At Vivoryon, we are passionate about creating small molecule medicines to improve the lives of all those who are affected by severe diseases. We build on our in-depth expertise in understanding post-translational modifications and pathologic pathways to develop medicines that modulate the activity and stability of proteins which are altered in disease settings in many different diseases. We are applying our expertise and technology to medicines to address the medical need in a number of severe diseases, focusing on inflammatory and fibrotic diseases.
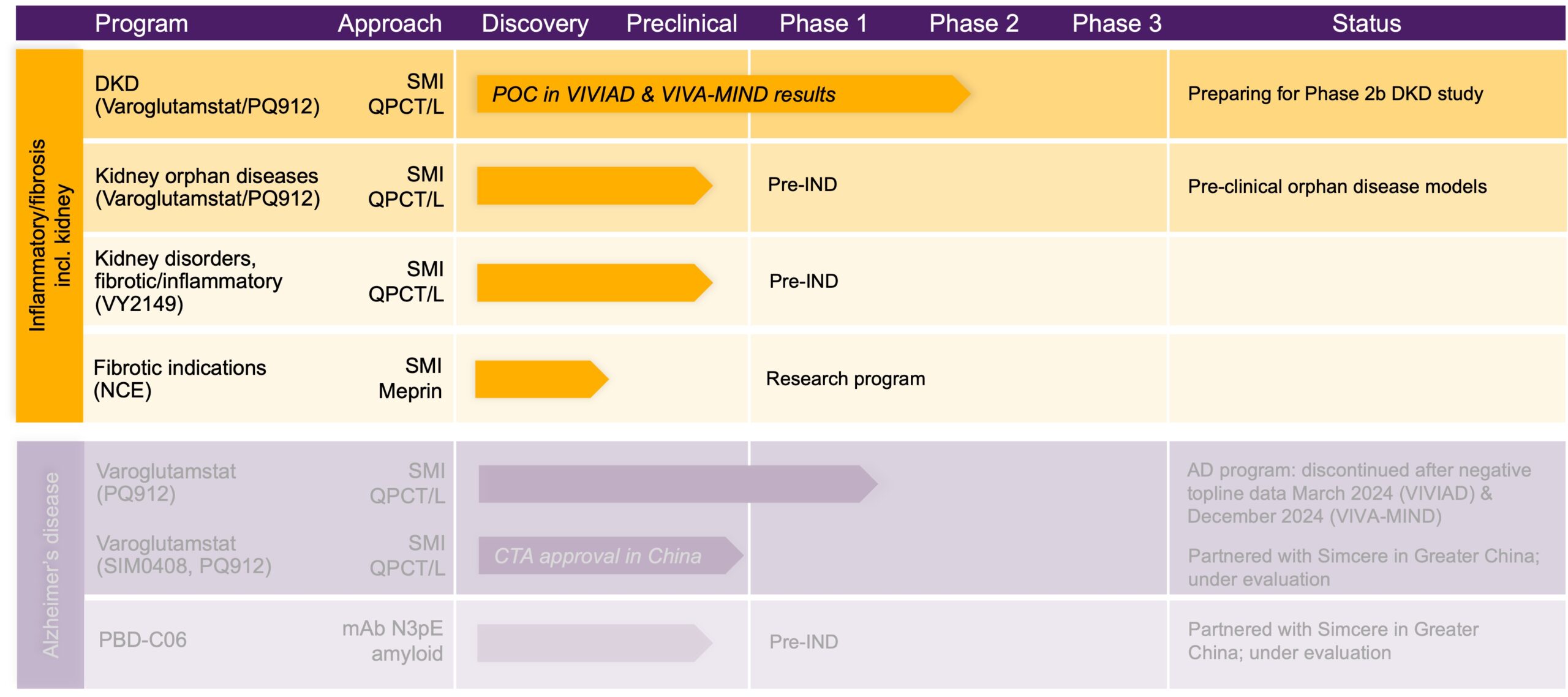
Pipeline
Developing a pipeline of small molecule enzyme inhibitors to treat severe diseases
We are applying our expertise and technology to create a diverse pipeline addressing severe indications including inflammatory and fibrotic diseases, neurological diseases, cancer and others. Our primary focus is on inflammatory and fibrotic disorders, including kidney disease.
Our most advanced research and development program targets the glutaminyl cyclases QPCT and QPCTL. These enzymes catalyze a specific post-translational modification process known as pyroglutamate formation, which is the formation of a ring at the N-terminus of a number of different peptides and proteins. This post-translational modification changes the properties of the proteins and the modified proteins have been shown to play important roles in causing or promoting a number of different diseases.
Blocking these enzymes to prevent the modifications enables an intervention early in the different pathologic pathways. We are developing a pipeline of orally administered small molecule inhibitors of glutaminyl cyclases, with our most advanced medicine in development, varoglutamstat, having demonstrated a beneficial effect on kidney function in two independent Phase 2 studies in an elderly patient population. Based on these findings we are focused on pursuing development of varoglutamstat in kidney disease.
Beyond QPCT/L inhibitors, we are assessing the potential of meprin inhibitors and also have programs to develop selected monoclonal antibodies (mAbs).
Varoglutamstat
Lead Candidate Varoglutamstat
Our lead candidate, varoglutamstat (PQ912) is a proprietary, potent (nanomolar) and selective inhibitor of human glutaminyl cyclases QPCT and QPCTL with therapeutic potential in indications including inflammatory and fibrotic diseases, neurodegenerative diseases, cancer and others.
Initially advancing development aiming to treat Alzheimer’s disease (AD), varoglutamstat has been investigated in a number of different clinical studies, all of which have consistently demonstrated a favorable safety and tolerability profile both in healthy volunteers and patients with AD. While no consistent effect of varoglutamstat in early AD was observed at the doses tested, we observed highly encouraging efficacy data from the two independent Phase 2 early AD studies (VIVIAD and VIVA-MIND) showing an improvement in kidney function as measured by a statistically significant increase in the estimated glomerular filtration rate (eGFR) in this elderly population. Based on this, we are focusing on developing varoglutamstat in kidney disease.
QPCT/L inhibitors in kidney inflammation/fibrosis:
Over a decade of research and know-how supports scientific rationale
Persistent low-grade inflammation is now considered a hallmark feature of chronic kidney disease (CKD)1
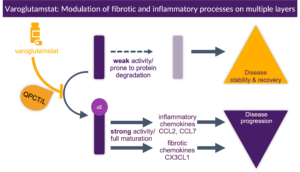
There is extensive scientific proof that many inflammatory and fibrotic pathways require formation of N-terminal pyroglutamates (pE) for full activity and the pyroglutamate (pE) versions of chemokines like CCL-2 and CX3CL1 (fractalkine) have been shown to be increased in chronic kidney disease (CKD) and may contribute to a number of renal diseases2,3,4,5
CCL2 is a validated target in kidney disease
QPCT/L inhibition has been shown to improve kidney function and reduce inflammation in a glomerulonephritis CKD rat model via CCL2/CCR2 axis, using a Vivoryon compound6 and CCL2 deficiency has been demonstrated to protect against chronic renal injury in murine renovascular hypertension7 . Blocking CCL2/CCR2 signaling ameliorates diabetic nephropathy in the db/db mouse model of type 2 diabetes8. CCL2 plasma levels were significantly higher in patients with CKD compared to the control group9.
-
1 Yilmaz MI et.al., Clin Nephrology 2007
-
2 Tesch GH, Am J Renal Physiol 2008
-
3 Kehlen et.al , Biosci Rep, 2017
-
4 Cynis et.al., EMBO 2011
-
5 Cynis et.al, Intl Jour. of Exp. Pathology 2013
-
6 Kanemitsu et.al., Naunyn-Schmiedeberg’s Arch. of Pharmac., 2021
-
7 Kashyap et al. Sci Rep. 2018
-
8 Soek et al. Nephrol Dial Transplant, 2013
-
9 Vianna et al., Pediatr Nephrol, 2013
Clinical proof of concept data:
Varoglutamstat improved kidney function in completed Phase 2 VIVIAD and VIVA-MIND studies
Based on the known anti-inflammatory activity of varoglutamstat, the protocol for the Phase 2 VIVIAD and VIVA-MIND studies in early AD, which were completed in 2024, included the investigation of kidney function and measurement of biomarkers of kidney inflammation and fibrosis to explore the role of QPCT/L inhibition on kidney function. Although patients in these studies were selected for their AD status and not for their kidney function level, many of them had reduced kidney function due to age and or comorbidities. The two independent Phase 2 clinical studies demonstrated a statistically significant improvement in kidney function (as measured by an increase in eGFR) under treatment with Vivoryon’s lead candidate, varoglutamstat. The treatment effect, in particular in patients with diabetes, was unprecedentedly large and was shown to be sustainable over a period of 2 years.

QPCT/L inhibitors in Alzheimer’s disease
Initially in development to treat Alzheimer’s disease (AD), a severe neurodegenerative disorder affecting around 30 million people worldwide, varoglutamstat targets the enzymes glutaminyl cyclases QPCT and QPCTL, which play an important role in promoting AD via QPCT-mediated formation of a neurotoxic Abeta variant, N3pE amyloid (pGlu-Abeta) and QPCTL-modulated CCL2 neuroinflammatory activity. Targeting these two enzymes, enables varoglutamstat to work upstream of other approaches such as monoclonal antibodies.
Varoglutamstat has been investigated in a number of different clinical studies, all of which have consistently demonstrated a favorable safety and tolerability profile both in healthy volunteers and patients with AD. While a first-in-patient Phase 2a study showed promising signs of efficacy after only 12 weeks of treatment, varoglutamstat did not meet its clinical endpoints in the Phase 2b VIVIAD study in Europe and the Phase 2a/b VIVA-MIND study in the U.S.
Based on these results, as well as highly encouraging efficacy data from the two studies, showing an improvement in kidney function under treatment with varoglutamstat, development of varoglutamstat in AD was discontinued and Vivoryon is now focused on developing varoglutamstat in diseases with an inflammatory / fibrotic component, such as of the kidney.
Expanded Access Policy
Vivoryon Therapeutics is committed to developing and commercializing safe and effective small molecule-based medicines which modulate the activity and stability of pathologically altered proteins for patients living with severe diseases with limited or no treatment options. We appreciate the urgency of bringing these innovative products to patients, and this commitment drives our work each day. We believe in the importance of collaborating closely with patients, their families, patient advocacy organizations, physicians, researchers and regulatory authorities to help achieve this goal. In these efforts, we are guided by ethical, scientific and legal principles, which require well-designed and controlled clinical trials to evaluate the safety and efficacy of our potential investigational medicines. Click here to visit the National Institutes of Health (NIH) website to learn more about clinical trials and how they work.
Our Approach to Clinical Development for Small Molecule-based Medicines
A robust and comprehensive clinical development program – designed with input from experts in the medical community, regulatory authorities and the patient community – is the optimal way to bring an approved therapy to the entire patient community. Clinical trials are designed and implemented to gain an understanding of the safety and efficacy of investigational new therapies in specific populations. Participation in clinical trials accepted by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), or other regulatory authorities, is the best way for patients to access investigational therapies for their diseases.
It is our hope and intention to quickly enroll eligible patients into clinical studies in order to demonstrate the safety and efficacy of our investigational oral small molecule medicines, and to rapidly obtain the global regulatory approvals needed to make these therapies widely available to the patients that need them around the world.
Expanded Access
Under certain circumstances, a person suffering a serious or life-threatening disease may ask to use an experimental treatment outside a clinical trial, before its safety and efficacy have been fully evaluated, and before the regulatory authorities have approved it. Generally, this is an option only for patients who have exhausted all available medical options and do not qualify for the ongoing clinical trials.
Vivoryon understands the intent of expanded access programs but, at this time, we can best advance the development of these potential promising products by enrolling patients in clinical trials which are designed to enable approval allowing access for the broader patient community. Our priority is to demonstrate the safety and effectiveness of varoglutamstat (PQ912), our investigational oral small molecule medicine in development to treat Alzheimer’s disease, in order to obtain regulatory approval and make it available to appropriate patients as rapidly as possible. We do this by running a thoughtfully designed and robust clinical trial program.
We are currently unable to offer expanded access for varoglutamstat (PQ912) and we believe that participation in one of our clinical trials is the most appropriate way to access our investigational therapies.
More Information
Treating physicians, patients and/or caregivers interested in learning more about Vivoryon’s investigational oral small molecule medicines currently undergoing clinical studies can find more information here or by or visiting www.clinicaltrials.gov and searching for Vivoryon Therapeutics.
If you are a health care provider who is interested in learning more about one of our investigational therapies, or a physician with questions about participation in one of our clinical trials, please submit a request to clinics@vivoryon.com. Vivoryon will acknowledge questions as soon as possible, usually within 5 business days of receipt.
If applicable, this website will be updated with hyperlinks to the relevant expanded access information on www.clinicaltrials.gov upon activation. As authorized by and in accordance with the 21st Century Cures Act, Vivoryon reserves the right to revise this policy at any time.
